Metabolic Acidosis
Introduction
Definition: A process whose primary event is a deficit in body bicarbonate stores. Characterized by a reduction in serum HCO3- concentration, ß in blood pH, and a compensatory ß in PaCO2 .
Note: Definition refers to a PROCESS. Can not conclude what the pH is from the term metabolic acidosis, only that there is an increase of arterial [H+].
Etiology: Acidosis is generated by either a gain of acid or a loss of HCO3-. The processes causing a gain of acid or loss of HCO3 - can be organized by the different effects on ECF electrolytes as measured by the "anion gap"
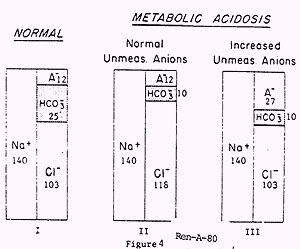
Anion Gap
important to determine to find cause of metabolic acidosis
Importance: helps differentiate the causes of a metabolic acidosis
Definition: represents Unmeasured Anions in serum: Phosphate, citrate (blood), sulfate, protein
Serum anion gap = [Na+] – ([Cl-] +[HCO3 -]
Normal value: 12mEq/L
Use: In Metabolic Acidosis (MA), the serum [HCO3-] ß as it is depleted in buffering fixed acid. For electroneutrality, the concentration of another anion must Ý to replace [HCO3 -]. That anion can by Cl- or an unmeasured anion. For this reason a [HCO3 -] ß can be manifested as either: normal or Ý anion gap
Etiologies
(1) Normal anion gap: Bicarb loss or Cl gain. Hyperchloremic Metabolic Acidosis
Causes:
- Renal tubular acidosis (RTA):
- Type I (distal):
failure to excrete titratable acid and NH4; failure to acidify urine; inability of tubule to eliminate H+; see hypokalemia and Ý urine Ph
- Need 2 things for proper H+ elimination in the distal tubule:
- buffers
(ammoniagenesis and titratable acids)
- pH low
enough so that the buffers can work (recall buffers operate optimally 1pH from their pK)
- e.g. Amphotericin B (anitfungal): punches holes in wall of distal tubule
Þ no H+ gradient Þ Ý urine pH (7) Þ buffers bind no H+ because pH is too high. Titratable buffers (Creatinine, sulfate) bind to K+ instead of H+ Þ hypokalemia
- Type II (proximal):
impaired proximal tubular HCO3- reabsorption; renal loss of bicarb
- Type IV
(hyperkalemic): aldosterone deficiency or unresponsiveness to aldo; failure to excrete NH4; hyperkalemia caused by lack of aldosterone
Massive diarrhea: GI loss of bicarb
Carbonic Anhydrase inhibitors: leads to loss of bicarbonate in the urine
Ammonium Chloride ingestion (ammonia synthesized to urea in the liver, leaving behind HCL)
Pancreatic drainage
Fistulae of small bowel
Treatment: Bicarbonate administration
(2) Increased anion gap: Ý concentration of unmeasured anions and hydrogen ion
- Causes
: results form either the production of an endogenous acid or the addition of certain exogenous compounds
- Endogenous acid:
- Lactic acidosis
(D or L type): L-type: tissue hypoxia, hypotension; D-type: associated with short-bowel caused by fermentation of incompletely digested carbohydrate by anaerobic bacteria.
- Diabetic ketoacidosis: generation of ketoacids from incomplete oxidation of fatty acids
- Alcoholic Ketoacidosis: starvation and ethanol accelerate ketogenesis
- Uremic acidosis: Severe Renal failure (GFR<20%) Not filtering phosphates from protein metabolism
Þ retention of unmeasured anions
Salicylate intoxication: interference with metabolic processes Þ accumulation of organic acids (lactic acid, ketoacids)
Exogenous compounds:
- ethylene glycol (antifreeze, radiator fluid)
- methanol (metabolized to formic acid)
- paraldehyde
Treatment: Treat underlying etiology (DKA with insulin). Treatment with bicarbonate is reserved for only severe acidemia (pH<7.1; bicarb<10mEq/L) because metabolism of organic anions (ketoacids, lactate) yeilds bicarbonate, and treatment with alkali may "overshoot" desired pH inducing a metabolic alkalosis. Also, NaHCO3 administration may induce iatrogenic hypernatremia, volume overload, Ý intracellular acidosis, cardiovascular compromise
CRF: failure of renal ammoniagenesis Þ impairs ability of kidney to rid body of excess H+; failure to excrete H+ as titratable acid and NH4
Compensation
Respiratory: Immediate but Never returns pH to normal
Metabolic acidosis is a process that causes a primary decrease in [HCO3 -]. The respiratory compensation (Kussmaul breathing) is an increase in the depth and rate of respiration which reduces alveloar and arterial pCO2.
- Max drop in pCO2 is down to 10mmHg (normal: 38-42mmHg). Below 10, Ý ventilation will Ý CO2 Þ ß pH rapidly
- PCO2 decrease of 10mmHg = pH rise of 0.08
- ß
1mEq/L [HCO3 -] = 1.0-1.3mmHG pCO2 ß
- If the magnitude of the respiratory compensation is inappropriate, suspect a mixed acid-base disorder
- PCO2 = 1.5 x (HCO3-) +8
- So, if the measured PCO2 differs from the this predicted value, a coexisting respiratory disorder is present because pCO2 is not behaving as expected.
- If measured pCO2 is higher than predicted Þ coexisting respiratory acidosis and vica versa
- Allow pCO2 to be +/- 2mmHg
Renal: excretion of ammonium and titratable acid (phosphate)

